Here, the case of single-stage distillation is presented. This is a simple case involving only boiling the mixture and then separating it in the column. Of course, neither plates nor packing is needed. Despite its simplicity, it is worth discussing the physics of the process.
 |
| Instantaneous evaporation |
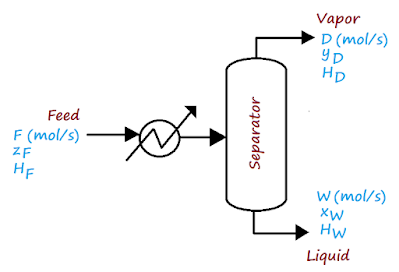
In the sketch above, a mixture feed $F$ is passed to a heat exchanger that boils the liquid and then to a separator. In the separator, the vapor rises to the top, while the liquid settles to the bottom. A mass balance for this very schematic case is twofold: a global balance and a balance for the component of interest (which may be more than one). For the global mass balance, we have,
$F=D+W$ Eq. (01)
and for the component of interest, the balance is written as,
$Fz_F=Dy_D+Wx_W$ Eq. (02)
An energy balance based on the enthalpy of each stream can be written as,
$FH_D+Q=DH_D+WH_W$ Eq. (03)
where $Q$ is the heat supplied in the heat exchanger to boil the feed F. Equations (01-02) can be combined to give,
$\dfrac{W}{D}=\dfrac{y_D-z_F}{z_F-x_W}$ Eq. (04)
which can be a more useful relationship. Also, Eqs. (01,03) can also be combined. The result is,
$-\dfrac{W}{D}=\dfrac{H_D-\left( H_F+Q/F \right)}{H_W-\left( H_F+Q/F \right)}$ Eq. (05)
This is the end. Enjoy.

No comments:
Post a Comment